This page contains automatically translated content.
Mutational diseases: Kassel and Krakow researchers decode protein complex
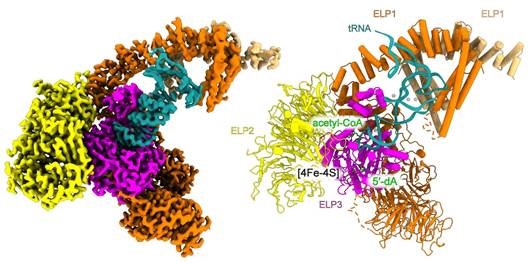 Image: Sebastian Glatt.
Image: Sebastian Glatt.The human body is constantly producing new proteins that are necessary for many essential functions. Certain molecules called tRNAs are of great importance for this production process. The elongator complex modifies sites on the tRNAs so that they can fulfill their task precisely. If errors occur due to genetic mutations, this can lead to serious diseases.
The Elongator is a protein complex that consists of a total of six subunits and is divided into two modules: ELP123 and ELP456. Prof. Raffael Schaffrath, Head of the Department of Microbiology, explains: "Our aim is to understand how the protein complex works in humans. The high-resolution structure of human ELP123 that has now been created enables us to precisely and directly determine the positions of the mutations that lead to Elongator-associated diseases."
"We were able to visualize the structure of the elongator complex at a resolution of 2.9 angstroms (Å) - the highest resolution ever achieved for this complex. The images show the elongator in complex with tRNA and an acetyl-CoA molecule, which provides important insights into the active center of the complex. A surprising discovery was the role of a neighboring uridine (a component of tRNA), which is necessary for the activity of the elongator. This finding was previously unknown and significantly expands our understanding of how the complex works," says Prof. Sebastian Glatt, head of the Max Planck Research Group at Jagiellonian University Krakow.
The high-resolution structure is an important step towards better understanding the effects of mutations on the elongator complex and developing potential treatments for diseases caused by mutational defects.
The study was recently published in Nature Communications: https://doi.org/10.1038/s41467-024-48251-y.
The research project was supported by the European Research Council, the Foundation for Polish Science and the German Research Foundation.
Contact:
Prof. Dr. Raffael Schaffrath
Head of the Department of Microbiology
Phone +49 561 804-4175
E-mail: schaffrath[at]uni-kassel[dot]de